Abstract
Hypercoagulopathy and hypofibrinolysis are both described contributors to glomerular disease-related thrombotic risk, however the factors contributing to the latter are poorly understood. The aim of this study was to investigate the pathogenic mechanisms underlying the purported hypofibrinolytic defect, using two well-established rodent models and a cohort of adult and pediatric patients with glomerular proteinuria. We hypothesized that hypofibrinolysis arises from derangements in plasma clot architecture that lead to increased fibrin density and altered incorporation of pro- and anti-fibrinolytic proteins into clots.
In vitro experiments were performed on two pathologically distinct rat models of glomerular disease exhibiting a wide range of proteinuria severity (n=5-8 per interquartile range (IQR) and experimental model), and a local cohort of pediatric and adult patients with glomerular proteinuria (n=23). Morning spot urines were analyzed for protein:creatinine ratio and citrated venous blood was collected and spun down to platelet poor plasma (PPP). Plasma albumin levels were measured by bromocresol purple assay. Fibrinolysis was initially confirmed by whole blood rotational thromboelastometry (rotem), and further assessed by plasma clot lysis assay (CLA) using urokinase (50 IU) to initiate lysis. Plasmin and thrombin generation were measured fluorometrically with their respective fluorogenic substrates. Clot fibrin network structure was visualized by laser scanning confocal microscopy using fluorescently-labeled fibrinogen as a tracer. Fibrinolytic proteins in plasma and clots were measured by ELISA and western blot, respectively. Potential fibrinolytic markers of interest were identified and further investigated by systematic plasma depletion (antibody-coated magnetic beads) or repletion (spiking with purified protein) and subsequent determination of clot lysis.
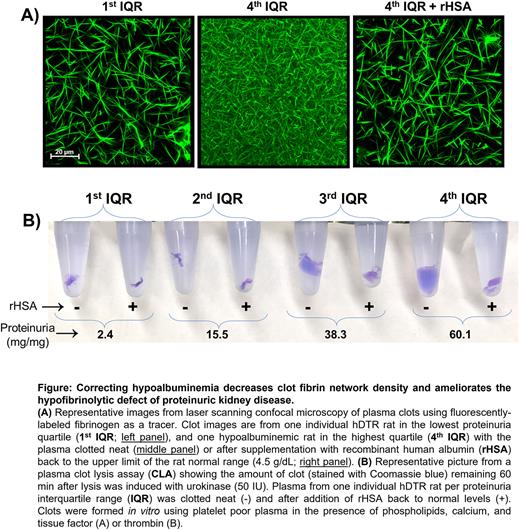
Clot fibrin network density and resistance to fibrinolysis were directly proportional to markers of glomerular disease severity (proteinuria and hypoalbuminemia; R≥0.462, P≤0.01) and hypercoagulopathy (fibrinogen concentration and thrombin generation (R≥0.637, P≤0.001). There was no correlation with uPA, PAI-1, α2AP, tPA, TAFI, or plasminogen, whereas plasmin generation was paradoxically increased in relation to disease severity. Incorporation of TAFI, PAI-1, α2AP, and α2-macroglobulin into clots was enhanced with increasing disease severity (P≤0.01), however depletion of these antifibrinolytic proteins to <10% of normal did not significantly improve clot lysis. In contrast, addition of recombinant albumin to upper normal levels (4.5 g/dL) restored fibrin network density and ameliorated the hypofibrinolytic defect (P≥0.957 vs control; Figure).
These data represent a comprehensive examination of the fibrinolytic system in the setting of glomerular proteinuria, revealing for the first time that albumin plays a significant regulatory role in fibrinolysis. Taken together these data strongly suggest that hypoalbuminemia is not just a biomarker of thrombotic risk but also contributes mechanistically to hypofibrinolytic pathology. Future studies will seek to understand the molecular mechanisms by which albumin enhances clot lysis may refine the use of albumin supplementation for persons at risk for life-threatening thrombosis due to proteinuric kidney disease or other hypoalbuminemic conditions.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.